DILUTING PERCENT SOLUTIONS & STOP BATH
I was thinking about how you calculate percent solutions the other day when discussing Glacial Acetic Acid mixtures to make stop bath. I knew I had seen an easy method, but I could not remember how it worked. Certainly it is fine to just follow directions, but what if you want to know how the process works, or you need to calculate some dilution other than the norm? I thought the equation was called the ‘X’ System or something similar. A search on the Internet did not turn up what I was looking for though. Seems things always come to me when I take a nap. . . this time I had to sleep on this one for several days before it came to me.
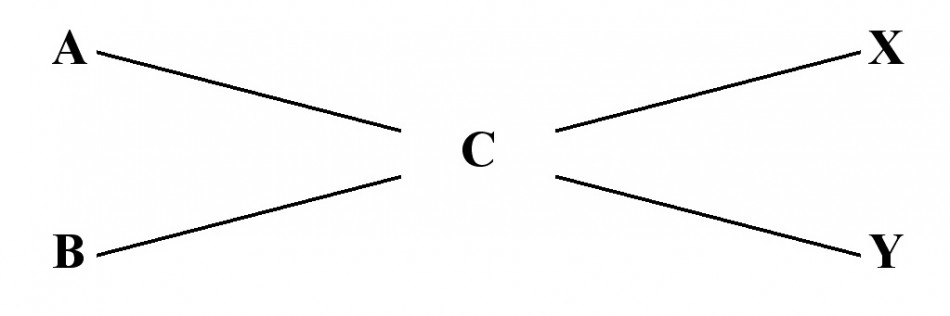
The simple procedure for calculating percent dilutions is called the Criss-Cross method and is really easy, if you can remember how it works. Once I had the right description, it was easy to find more information, and it is really quite simple.
Here is the Criss-Cross formula;
To work the Criss-Cross formula do the following:
A = the % dilution of the solution to be diluted
B = the % dilution of the diluting solution (for Water this value is Zero)
C = the % dilution desired
X = C – B
Y = A – C
Diluting X parts of A with Y parts of B will yield a % solution equal to C.
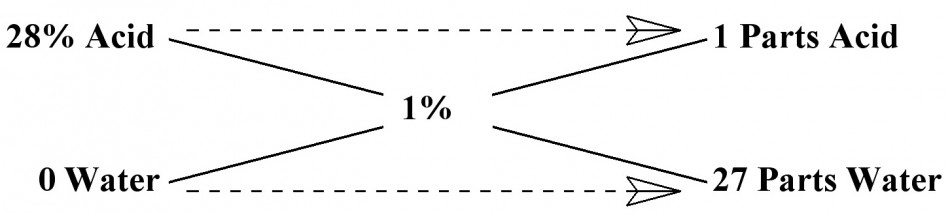
I know it all sounds complicated and it really is much more difficult to explain than it is to actually work the problem. So, lets go through an example that hopefully will make it more understandable. Most all agree that an acid stop bath should be somewhere between a 1-2% dilution of Acetic Acid in water. I have always used a 1% solution and that is what we still use today. For example, let’s dilute 28% Acetic Acid stock to a 1% mixture for stop bath. Plug in the correct values, then perform the calculation. Enter 28% for term A, 0 for term B, 1% for term C, then perform the calculation.
You will find that if you mix 1 unit of 28% Acid with 27 units of Water you will get a 1% solution for your stop bath. Remember, we are working with ratios and the Units can be anything desired, as long as they are the same Units. It could be a mixture of 1:27 ounces, gallons, milliliters, liters, whatever units you desire. You can change the values of X and Y if you want. Just keep in mind that you have to change both Units by the same amount. If you multiply the ratio of 1:27 by 2, you would have a ratio of 2:54 Units. You could also divide the Units for smaller volumes.
For the sake of a working example, if you multiply the ratio of 1:27 in the example above by 40, you get a ratio of 40:1,080. This is how I dilute 28% Acetic Acid to a 1% solution for paper stop bath. I use even numbers of 40:1,000 milliliters. Plenty close enough for photography.
Hope this helps. . . it is not difficult if you can remember the formula.
JB
MY REPLACEMENT FOR ZONE VI PRINT & FILM FIXER
 I had written earlier about the demise of Zone VI chemicals and how it has now become necessary to mix our own chemistry from raw supplies. Take a look at the previous post titled, “ROLLING YOUR OWN FIXER.” Since that post I have done quite a bit of research and testing. I am convinced that the fixer formula we chose is not exactly a direct replacement for the old reliable Zone VI product. Not that what we have been using is not a good fixer, it just is not the same. Fred always said, “Different is not the same.”
I had written earlier about the demise of Zone VI chemicals and how it has now become necessary to mix our own chemistry from raw supplies. Take a look at the previous post titled, “ROLLING YOUR OWN FIXER.” Since that post I have done quite a bit of research and testing. I am convinced that the fixer formula we chose is not exactly a direct replacement for the old reliable Zone VI product. Not that what we have been using is not a good fixer, it just is not the same. Fred always said, “Different is not the same.”
I have mixed up several concoctions, but have always come back to the Looten Acid Fixer for its simplicity and close match of pH. I kept bypassing some of the more well known formulas for some reason, possibly because everyone says they have a very unpleasant odor. I don’t believe any could be any worse than the Looten Acid Fixer we have been using. . . this stuff will take the hair out of your nose.
I want to use an acid fixer for paper, because that is what I have used for years. All I wanted to do was find a 100% replacement for the Zone VI Fixer I have used for as long as I can remember. Zone VI Fixer was stable and it had little to no odor. I have searched all over and have never found the exact published formula used by Zone VI.
I looked through all of the old Zone VI Newsletters and finally resorted to watching the Picker videos. Finally in the printing video Fred talks about his fixer being Kodak F-6. The only problem is that the Zone VI Fixer I have used for years always came in a single bag as dry chemical. If you check the formula for F-6 you will find it contains 28% Acetic Acid. I am no chemist, but as far as I know, 28% Acetic Acid only comes in liquid form. Plus, F-6 is a hardening fixer. I have no reason to believe that Zone VI is a hardening fixer, let alone all instructions for F-6 say that the hardener, Potassium Alum, must be dissolved separately and added after all other ingredients have been completely dissolved. Again, the Zone VI Fixer came in one bag containing only dry chemical. So, I really do not believe that F-6 is the correct formula for what was sold as Zone VI Fixer.
There are a lot of guesses, but I do not want a guess. I measured the pH of Zone VI Fixer to be 5.5. None of the other concoctions that I have tried matched this pH, nor did they lack a strong odor. My original choice of Looten Acid Fixer had the closest pH coming in at 5.0, but has a strong odor, where the Zone VI Fixer has little odor at all. I still found myself going in circles.
I finally got tired of having to run the vent fan on high to get away from the smell. Time to put on the apron, roll up the sleeves, and do more research. This time I decided to investigate the Kodak F-24 formula. Why I bypassed this one before is beyond me, but I did, and it was a mistake. There is an alternative mixture that is suppose to be ‘low-odor’ that substitutes Citric Acid for the Sodium Bisulfite of the original Kodak formula. This mixture still had a very strong smell. So much for the alternative, low-odor mixture!
Next, I mixed up a two liter batch of the original F-24 formula, and surprise. . . it smells just like Zone VI Fixer. Next to no odor at all. I measured the pH. . . well whaddya know. . . it was exactly 5.5, just like Zone VI. Next I needed a stability test. I put two liters of F-24 in a four liter jug and let it set for a week. No change! That does it for me. So far as I am concerned, there is no difference.
Wow! Have I solved the great mystery? Could it be that Zone VI Fixer is nothing more than Kodak F-24? I have changed our FORMULAS AREA to reflect the formula for F-24 Fixer since it is now our chosen formula for paper. Note, that the only difference in the new formula is the addition of 10 grams of Sodium Sulfite per liter to the original Looten Acid Fixer formula. Not that difficult, but it does make a difference. Even if F-24 is not the exact same formula as Zone VI, it is plenty close enough for me. I will add that Gordon Hutchings also recommends F-24 for film and paper in “The Book Of Pyro.” So, now you know what I have learned about fixer.
There is one more thing to be aware of; be very careful with the temperature of the water when mixing Sodium Thiosulfate. The most common Sodium Thiosulfate is the Pentahydrate (crystalline type) which requires 240 grams per liter. You need water at about 100-125°F, because it is extremely endothermic and will rapidly cool the water as it dissolves. This I knew from experience. What I didn’t know is that Sodium Thiosulfate Anhydrous (fine grain like table salt), which requires 152 grams per liter, should never be mixed in water above 90°F, because it will decompose and form a precipitant. Just so you know, if using Sodium Thiosulfate Pentahydrate (large crystals) use hot water. If you are using the Anhydrous (fine grain) type, mix at about 80-85°F.
CASE CLOSED. . . at least for me. I have found what I was looking for. If you were a Zone VI Fixer user and are looking for a suitable, easy replacement, this should work just fine. Now, back to making photographs. . . and. . . I can turn that vent fan down to low again!

JB
Kodak F-24
- Water (at about 125°F)…………………750.0 ml
- Sodium thiosulfate, crystalline………240.0 grams
- Sodium sulfite, desiccated……………10.0 grams
- Sodium Bisulfite………………………..25.0 grams
- Water to make……………………………1.0 liter
Note: If anhydrous thiosulfate is used, the water temperature should not be over 90°F (80-85°F) to prevent decomposition.
- Water (at about 125°F)…………………750.0 ml
- Sodium thiosulfate, crystalline………240.0 grams
- Sodium sulfite, desiccated……………10.0 grams
- Sodium Bisulfite………………………..25.0 grams
- Water to make……………………………1.0 liter
Note: If anhydrous thiosulfate is used, the water temperature should not be over 90°F (80-85°F) to prevent decomposition.
PYRO STAINING FILM DEVELOPER
 I had written about my experiments with Pyro Staining Developers back in October of 2010, and that should make it very clear that I have chosen my favorite film developer. Take a look at my previous post, “THE PYRO-CAT IS OUT OF THE BAG.” Of all of the Pyro Staining Developers I tested, PyroCat-HD by Sandy King has proven to be, hands down, the best of the best. If you are seriously looking for the finest all-around film developer, I would strongly suggest you take a serious look into PyroCat-HD.
I had written about my experiments with Pyro Staining Developers back in October of 2010, and that should make it very clear that I have chosen my favorite film developer. Take a look at my previous post, “THE PYRO-CAT IS OUT OF THE BAG.” Of all of the Pyro Staining Developers I tested, PyroCat-HD by Sandy King has proven to be, hands down, the best of the best. If you are seriously looking for the finest all-around film developer, I would strongly suggest you take a serious look into PyroCat-HD.
Sandy King has done his research and formulated an excellent developer. Also, he has a new web site that is outstanding. I was pleased to find that he has a section dedicated to his technical writings which contains his original article on Pyro Developers. If you are seriously thinking about working with Pyro Developers, I would highly recommend you take a look at the article, “AN INTRODUCTION TO PYRO STAINING DEVELOPERS, WITH SPECIAL ATTENTION TO THE PYROCAT-HD FORMULA” by Sandy King.
There is no need for me to go into the details of my experiments with Pyro Developers since I have covered what I learned in my previous BLOG post. I would also recommend the above mentioned article by Sandy King, which covers a lot of what you need to know about Staining Developers. All I can add is that PyroCat-HD is the only developer we now use. Maybe you should try it yourself?
JB
WEIGHT BAGS FOR MOUNTING PHOTOGRAPHS
 Like most everything I do when it comes to photography, I am not the source of the technique, idea or clever device. I would say that 95% of what I do is something I learned from someone else. I may add my own twist, but I cannot take credit for the idea.
Like most everything I do when it comes to photography, I am not the source of the technique, idea or clever device. I would say that 95% of what I do is something I learned from someone else. I may add my own twist, but I cannot take credit for the idea.
Mounting photographs is a tedious and time-consuming task that we all must master if we want to display our photographs. Dry mounting is our chosen method of print presentation. Anyone that has dry mounted photos knows what is involved. You tack dry mount tissue to the back of the photo, trim the edges, then align the image on the mount. It is, at best, a nerve racking chore and one slip then you have just ruined a print.
It is imperative that you get the photograph aligned properly on the mount. This is a tedious process of measuring and measuring again. . . and. . . maybe you should measure again! Nothing is more frustrating than when you get everything just right, you bump the print and have to start again. What you need is something to hold the print in place while you do the final tacking to the mount. This is where a weight bag comes into play. For a small print only one small weight bag should be enough. With larger prints a larger weight bag might be better, or possibly two smaller ones would be better to hold things in place. Either way, a good weight bag is a big help.
So, now that we have solved the problem of what to do, we need to look at how we can construct a suitable weight bag. For me, simple is always better. . . inexpensive is even better than simple. So here is how I solved the mounting weight bag predicament.
A weight bag for mounting photographs must be made of some very soft material so as not to damage the print surface. The perfect material is a synthetic Naugahyde-type material that lens bags are made from. A good place to look for lens bags is your local camera show. There is nearly always a box under some table full of such things that range from really cheap to even free. What you are looking for are the bags that are made of very soft, supple material. A lot of the bags are made of very stiff, rough material that will damage a print. I have several size bags ranging from those that were intended to carry large, long, lenses, to those that would hold a small normal lens. See if you can find several large bags and a couple of the smaller ones.
Once you have the bags it is only a matter of stuffing them with a suitable weight material. I have heard suggestions from sand to marbles. I would suggest using Aquarium Gravel from the local pet supply as a great weight material. Any of these items should work well, but I did something completely different. First, I just don’t like the idea of rocks, sand or gravel in or around my work area. Second, I did not have that many marbles (some say I lost all of my marbles years ago). There was one thing I did have an abundance of and that was pennies. I had several large jars filled with pennies in the back of a closet. Why not use them, since they are heavy and should work well for my purpose.
No matter what material you choose for filler, I would highly recommend that you first load your weight material into a heavy plastic bag. I would even further suggest that you double bag your weight material, just in case. You will have to adjust the amount of filler needed to fill the lens bag and still allow you to close the drawstring end closed. Once you have the correct amount of filler in a plastic bag, close them securely with twist ties, or use a heat sealer. Note: Do not stuff the inner bags too full. You want them to be about 90-95% full.
All that is left to do is stuff the bagged weight material into the lens bag and close the draw strings. I tie the drawstrings off then cut off the excess.
I have had my weight bags, stuffed with pennies, for years and they have served me well. I use them for print mounting and for weight when flattening prints under a sheet of 1/4 inch glass. Weight bags are a handy tool to have around. The next time you are at your local camera store or a camera show, look for used lens bags. Pick up a few soft bags and make your own weight bags.
JB

ZONE VI DEVELOPING TIMER PROBE BRACKET
 I am a firm believer that necessity is the mother of invention. Let’s face it, most people do not design and build just for the enjoyment of designing and building. . . at least I don’t. Many years ago I decided to purchase the Zone VI Compensating Developing Timer and I have used it ever since. This is a great darkroom tool, and I would not process film nor paper without it.
I am a firm believer that necessity is the mother of invention. Let’s face it, most people do not design and build just for the enjoyment of designing and building. . . at least I don’t. Many years ago I decided to purchase the Zone VI Compensating Developing Timer and I have used it ever since. This is a great darkroom tool, and I would not process film nor paper without it.
The biggest problem I had when I first got my timer was trying to come up with a way to mount the sensor to my trays. Since by this time in my photographic career I was tray processing sheet film, I had no use for tanks and what was sorely missing was some sort of bracket to hold the sensor in the tray. I tried tape and even a plastic close pin. Nothing seemed to work, so it was off to think a bit. I do my best thinking while taking a nap.
After some time contemplating the situation, I finally visualized a clamping device that could be attached to the lip of a tray. I decided that it needed to be made of a tough material that wasn’t affected by chemistry and would be easy to keep clean. So I chose to make my clamp from a chunk of Acrylic.
I dug out an old hunk of scrap Acrylic from the junk box and began working it into shape with a band saw and vertical mill. What I eventually came up with was a prototype temperature clamp that I have been using for over ten years now. Take a look at the accompanying photos and you will see a very well used piece of darkroom equipment.
I have always wanted to improve a little more on my original design, but just never got around to it, until now. Though my prototype shows no signs of degradation, it is well stained from the Amidol days and my biggest concern is that there might come a time when the Acrylic would fail. You know how nothing ever fails except when you really need it. I could envision this thing breaking in the middle of a printing session or even worse, in total darkness during a film run. Though from careful examination of the original, I can see no signs of failure. Still, I always like having a spare on hand.
So, having decided I need a spare clamp it was time to do a little redesign and then off to the shop to cut up some Acrylic. I beefed up the clamp a little and reworked it so it could be drilled for both sizes of probes that came with the original timer. Yes, we do have a spare timer also. The only thing is the spare is the newer unit and uses the larger probe. My original Zone VI Compensating Developing Timer came with a probe with an OD of 0.1875 inches, while the newer model has a probe diameter of 0.250 inches.
As with most projects of this type, it is just as easy to make several as it is to make one. I cut up all of the Acrylic that I had on hand and made a handful of Probe Brackets. I still have some hardware on order, but I already feel better knowing I have spare parts on hand.